Effective & Easy
TMS is an effective treatment for depression. Just a few weeks of daily, short 20-minute sessions is all it takes. We have seen outstanding results in our clinic with the personal attention to the needs of each client by our TMS technician. Dr. George directly supervises each patient’s care. TMS is FDA approved and covered by insurance for treatment resistant depression. Click the button below to schedule a free consultation to see if TMS is right for you.

Why Choose Brighter Day TMS Clinic
- We have been serving patients in Fort Collins for over four years and performed over 4000 treatments.
- Dr. George is dedicated to the clinic and provides personal attention to each patient.
- Our TMS technician works one on one giving TMS treatments and Dr George monitors daily progress.
- Our results exceed those published in TMS studies with over 60% achieving remission of their depression and over 90% seeing significant improvement.
- Brainsway Deep TMS was selected by Dr. George because it provides the deepest and broadest stimulation of the brain area with depressed function.
- Brainsway has a unique patented coil unlike any other TMS device on the market.
- Our clinic is the only local clinic providing Brainsway Deep TMS.
TMS Press
proven results
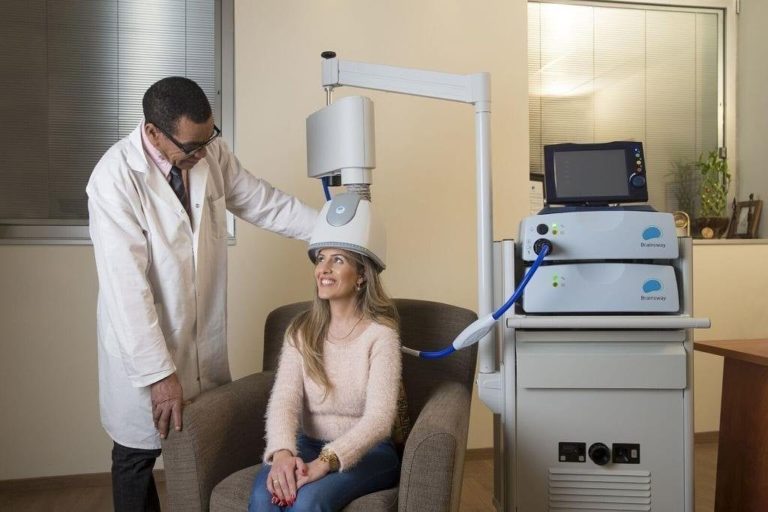
Tried & Trusted
Hundreds of centers across the globe trust and use Brainsway Deep TMS, including the following:








Frequently asked questions
Brainsway deep TMS is a non-invasive procedure involving stimulation of a part of the brain called the Left Prefrontal Cortex. The stimulation activates the nerve cells in this region of the brain and improves depressive symptoms. The stimulation is produced by a treatment “coil” contained in a helmet. During the treatment, the helmet is gently positioned on the left front side of your head, over this region of the brain. Short pulses of electricity sent through the treatment coil generate magnetic fields that turn on and off very rapidly. These magnetic fields are similar to those used in magnetic resonance imaging (MRI) systems. The magnetic field goes through the head and induces a weak electric current that briefly activates the nerve cells in this region of the brain and improves depressive symptoms. Read More.
TMS is covered by most major insurance companies for patients who meet insurance requirements for treatment resistant depression (have
already tried medications). Our in-network insurances include Blue Cross Blue Shield, United, Aetna, Cigna, TriCare, and Medicare. We may be able to work with other insurances on a case by case basis.
In clinical practice 3 out of 4 patients improve. 51% of individuals treated with TMS achieve remission and 75% of individuals treated improve (improvement means at least a 50% reduction in symptoms). This is remarkable considering these individuals have already tried 4 medications and a significant course of therapy.
Brainsway deep TMS is indicated for the treatment of depressive episodes in adult patients suffering from Major Depressive Disorder who failed to achieve satisfactory improvement from previous anti-depressants medication treatment in the current episode.
Brainsway deep TMS delivers a magnetic field that could cause any metal objects that are near the device to move or to get hot. The treatment should not be used if you have metal implants in or around your head (except for standard amalgam dental fillings). Brainsway deep TMS should not be used if you implanted electronic devices in your body. These implants could cause serious injury or death if Brainsway deep TMS is used. You should tell your doctor if you have any metal devices or objects in your head or body in order to determine if those devices could be affected by the treatment.
Brainsway TMS was approved for the treatment of obsessive-compulsive disorder (OCD) by the FDA in 2018. TMS is done along with OCD exposure response prevention therapy to provide successful reduction in OCD and improve a patient’s quality of life. Insurance coverage for TMS treatment for OCD is uncommon, but the exposure response prevention therapy is generally covered and helps reduce the cost. Read more.
TMS has been successful for treating bipolar disorder, anxiety, addiction, and other mental illnesses. The treatment involves magnetic
stimulation of brain structures and networks to the appropriate areas of the brain and can bring significant improvement to patients. However, these applications are not yet FDA approved and thus are considered an off-label use, so are not typically covered by insurance. Read More.
The safety of Brainsway deep TMS was demonstrated in a clinical study involving 233 patients with moderate to severe Major Depressive Disorder. The patients ranged in age from 22 to 68 years old. Long term safety has been demonstrated in a clinical study. During the full course of 16 weeks of ongoing treatment, the therapy was safely tolerated. Furthermore no negative effects of treatment were seen during a 3 month follow-up period. Longer term effects of the exposure to the treatment are not known. However, exposure to other devices (such as MRI scanners) with the same type and strength of magnetic fields produced by Brainsway deep TMS coil are not associated with significant short-term or long-term safety concerns.
There was one case of a seizure reported in the above mentioned safety study. This seizure was due to high alcohol consumption during the night before the treatment. 3 more seizures out of 50,000 treatment sessions were reported in other studies using Brainsway deep TMS in subjects on high doses of antidepressants which are known to increase the risk of a seizure. None of the people who have experienced Brainsway deep TMS induced seizure during the studies have suffered lasting physical effects. You should discuss with your doctor if you have had a seizure, or if you have a medical condition or change in a medical condition which may put you at increased risk of having a seizure, i.e. brain injury, change in medications, change in electrolyte balance, ect. Your doctor will decide if it is appropriate for you to receive Brainsway deep TMS treatment.
There are reports of headaches, discomfort from a tapping sensation, and jaw discomfort in some patients. During TMS a clicking sound is emitted. Patients must use earplugs. There have been no reports of hearing loss after the treatment during the clinical study when earplugs were used. Usually, these issues decrease or go away altogether with successive treatments. Let the technician know as adjustments can be made in the helmet position.
There were no deaths, no systemic side effects such as weight gain, dry mouth, and sexual problems and no decline of memory function during treatment and many people report improved clarity of thinking.